Neon electroporation device chickens out
UPDATE: We are not the only ones that try to economize on our running costs. This lab published its experiments in with the Neon system in Analytic Biochemistry. Thanks to Joachim Goedhart (@joachimgoedhart) for bringing this to our attention!
The Neon transfection device from Life Technologies (oops, Thermo Fischer nowadays and former Invitrogen) was introduced about five years ago to the market. It is designed for easy electroporation of mammalian cells. We have had the device available since 2011, but it was not much in use. I don't know whether the low adoption rate is due to the user-unfriendliness (I still don't know how to put the electrode tip to the pipettor despite having done this hundreds of times, it's just really finicky mechanics), expensive running costs (for its desposible gold-plated electrodes and the proprietary transfection buffer) or something else I cannot figure out.
However, there are a few things that I wanted to share because real useful information about the Neon device is scarce on the web.
The first thing that we had constant problems with were air bubbles in the electrode tip, which result in desastrously low electroporation efficiencies. The only way to really prevent this is to prepare at least 25% more cell suspension than actually needed. When you prepare only 10% more, the last electroporation will certainly arc due to unaviodable air bubbles (the cell suspension additionally sticks easily to the outside of the pipette tip which contributes to the need to prepare more than actually needed). As a consequence of this, we ran out of electroporation buffer R long before we ran out of pipette tips. Additionally one cannot purchase buffer R separately. The Life Technologies representative with whom I corresponded promised to send us a small batch of pipette tips/buffer in good will (that was in January), but we are still waiting for that to arrive...
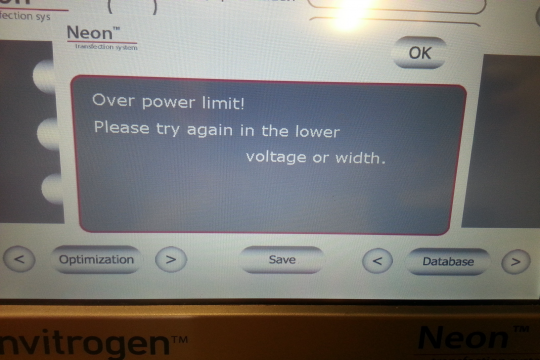
Unforatunately, recently our old Gene Pulser II electroporation device broke. Bio-Rad doesn't repair it anymore and also doesn't provide spare parts. It was mostly used for E. coli electroporation. I knew that the Neon device was not designed to electroporate E. coli. Why not and why can't it be used for that purpose? It turns out that the electric field strength is by far not enough for E.coli (in the BioRad Gene Pulser II, a typical bacterial electroporation achieves an electrical field of 12.5kV/cm, whereas the Neon barely achieves around 850V/cm. However (I thought), the neon can do much longer pulses than the Gene Pulser II (Neon is advertised to be able to give pulses up to 100 ms, whereas the typical pulse length of the Gene Pulser II is about 5 ms). In addition to this, the Neon can deliver automatically multiple pulses. So I wanted to test whether a long and/or repeated pulse with lower field strength can transform E. coli. However, it appeared that when you use water (or 10% glycerol) as the E. coli transfection buffer (which you usually do), the machine complains the tip electrode doesn't make contact. This is due to the fact that the machine tests whether you have inserted the tip correctly by sending a small current through the system and that current doesn't flow if you have resuspended your E. coli in water. In order for the machine to "accept" an inserted pipette tip electrode, you need somewhere between 10 and 20 mM NaCl. So I used 15 mM sodium chloride as E. coli electroporation buffer and set the electroporation parameters to 2500V and 100 ms. Surprise: The machine refuses to give such pulse because it is "Over power limit!". The maximum pulse length it can deliver with 2500V is 19 ms. Unfortunately even that pulse cannot be given automatically multiple times (again: "Over power limit!"). Therefore I manually executed this pulse between 1 and 20 times, but not a single bacterium received any DNA and all bacterial plates remained blank.
The manual states:
"The Neon TM device is designed to only input certain values and limits for each value are listed below. If your input value exceeds the maximum value, an error is displayed.
Input Voltage range: 500–2,500 V
Input Pulse Width range: 1–100 ms
Input Pulse Number range: 1–10
Unfortunately, this can be very easily misunderstood. It was not clear to me that one cannot combine the three parameters within these ranges freely. One should think that Life Technology has better technical writers (but maybe they don't use the device...)
Bottom line: The device is utterly useless for anything but mammalian cells. Also some other interesting applications (e.g. electroporation of nematodes or other small critters) are difficult or impossible. While the machine might be a good choice for many mammalian cells, it's much more limited than the old-fashioned BioRad Gene Pulser II.
P.S.: I used the buffer E to fill the pipette station (for use with 10 µl tips). However, I also tested instead of buffer E a mixture of 90% 150 mM sodium chloride and 10% glycerol (which gives me the same conductivity as buffer E has). However, I still really would like to know the composition of buffer R. Why? Because I think that the tip electrodes can be recycled more often than only twice (other manufacturers of pipette tip electrodes advertise that their electrodes can be recycled many more times (e.g. the BactoZapper, although they don't give precise numbers either). The Neon manual states that "Oxide formation at the piston surface area can be generated if the tips are used more than 2 times, which decreases electrode function of the piston." The electode is gold plated and gold should be more resistant to oxide formation than the stainless steel electrodes of the BactoZapper...