Stripping
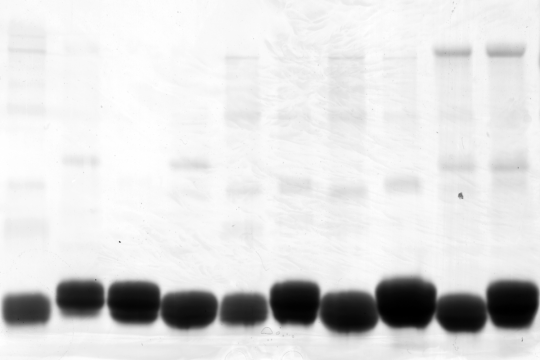
Reprobing membranes with with a different antibody is a very common task in the lab. Various protocols exist to strip membranes and the classic method is the one that uses SDS, β-mercaptoethanol and heating. I used to do it that way, but it's a smelly business, because β-mercaptoethanol smells like rotten eggs. Then suddenly everybody in the lab started to use the Re-Blot Plus Solution from Millipore and so did I. Until I realized by chicking the Material Safety Data Sheet that they sell cheap chemicals for a premium price. Now I make the stripping buffer myself. My 10x solution has the following composition:
- 2% sodium azide
- 1 M Tris/HCl pH 6.8
- 1.5 M NaOH
I use the diluted (1x) solution for 20-30 minutes at room temperature, wash the membrane 3x5minutes in TBS-T 0.2% Tween and block 2x30 minutes with 5%BSA in TBST or PBST 0.1% Tween.
However, the sodium azide doesn't strip, but irreversibly inactivates the horseradish peroxidase (HRP). Therefore, when you check the stripping efficiency, you might no see any signal with your chemoluminescence solution, but nevertheless there might be still bound antibody.