KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D
Prostate-specific antigen (PSA) is well known - at least among older males - as a prostate cancer marker, but few people know its physiological function: Sperm cells are trapped in fresh ejaculate, which has a jelly-like consistence. In order to release the sperm cells, the ejaculate needs to be liquefied and precisely this liquefaction is the task of PSA.
Also surprising for many people is the fact, that scientists still do not know why high PSA levels are associated with prostate cancer. In our latest research published yesterday in eLIFE, we have made a big step ahead in understanding the role of PSA in both reproductive and cancer biology.
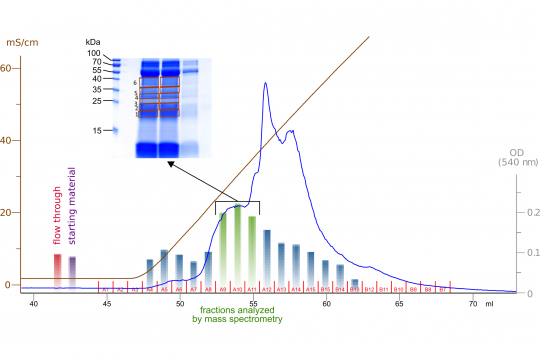
It appears that PSA (aka as kallikrein-related peptidase 3 - KLK3) and another enzyme called cathepsin D can activate two growth factors which have been implicated in cancer progression: VEGF-C and VEGF-D. These growth factors do likely contribute to tumor angiogenesis and tumor lymphangiogenesis. By inducing angiogenesis - the growth of blood vessels - the tumor ensures its own supply with nutrients and oxygen. Such blood supply is necessary for a tumor to grow beyond the size of a few millimeters. Likewise, tumor lymphangiogenesis happens when the tumor induces the growth of lymphatic vessels and it is tightly linked to the lymphatic spread (metastasis) of the tumor.
Both VEGF-C and VEGF-D are produced as inactive precursors (pro-VEGF-C, pro-VEGF-D) and need to be activated in order to induce the growth of blood or lymphatic vessels. With ADAMTS3, we have identified the enzyme that activates VEGF-C during embryonic development - which also requires vessel growth - in 2014 (Jeltsch et al.). However, it remained unclear whether the same enzyme is responsible also for pathological vessel growth. Now it seems likely that patholigical vessel growth uses different enzymes and PSA and cathepsin D have become prime suspects. Our next experiments will test whether we can slow down or halt cancer growth by blocking these enzymes.